Atom
n., plural: atoms
[ˈætəm]
Definition: The smallest possible unit of an element that still has all the chemical properties of that element.
Table of Contents
In the enchanting tapestry of life, the smallest of heroes and a celestial protagonist emerges—’the atom’. With grace and finesse, it takes center stage as Biology’s most captivating partner. Like microscopic architects or masterful artisans, atoms intricately weave the fabric of life, crafting molecules of paramount significance.
Atoms play a crucial role in the biological world as they form the basis of our existence. From constructing the intricate strands of DNA that define our genetic makeup to possessing the remarkable ability to transmit signals in our nervous system, catalyze chemical reactions, and provide the energy necessary for our bodily functions, atoms play indispensable roles in the functioning of the biological world. Understanding atoms is key to unraveling the mysteries of biology and gaining insights into the marvels of life. They may be tiny, but their significance in the biological realm is monumental.
Read on to learn how atoms constitute the bodies of biological significance and the different roles they play!
What Is An Atom?
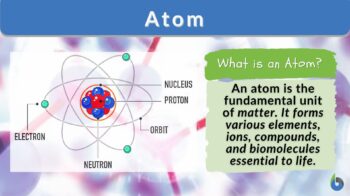
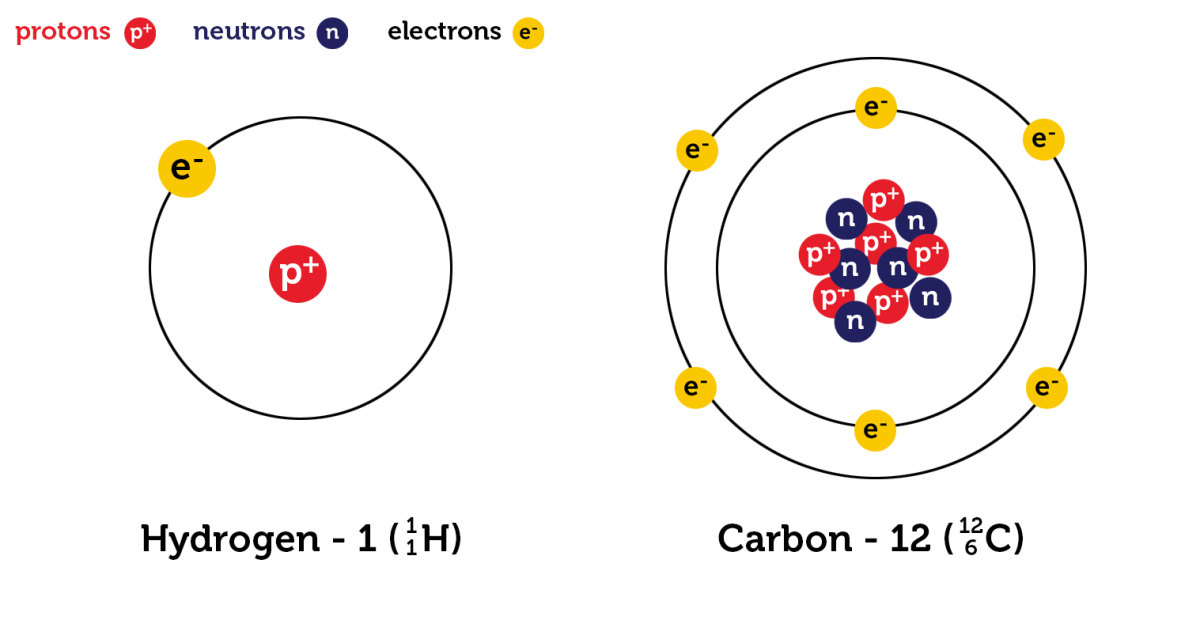
An atom is defined as the smallest unit of matter indivisible by chemical means. It constitutes the fundamental building block of a chemical element. In other words, an atom can be called the smallest possible unit of an element that still has all the chemical properties of that element.
-
How is an atom built?
An atom is built from an atomic nucleus surrounded by one or more shells of electrons. The nucleus is constituted by protons and neutrons.
-
Atom Etymology
The word atom is derived from the Greek word ‘átomos‘ meaning ‘uncuttable’ or ‘something that cannot be divided further’.
Watch this vid about atoms:
Biology definition:
An atom is the smallest unit of matter that cannot be divided by chemical means. An atom represents the elemental essence of matter, forming the intricate fabric of the physical world and playing a pivotal role in the diverse interactions and transformations observed in chemistry and the natural world.
Etymology: from Ancient Greek ἄτομος átomos, meaning indivisible.
-
Atom: Relevance in Biology
Studying atoms is of paramount relevance for biologists due to their fundamental role in understanding the intricacies of life. Atoms form the basic constituents of molecules, including essential biomolecules like proteins, DNA, and carbohydrates. By comprehending the arrangement, properties, and interactions of atoms, biologists can unravel the structure-function relationships of these molecules, deciphering how they contribute to the diverse phenomena observed in living organisms. Moreover, atoms are involved in chemical reactions and energy transfer processes within biological systems, shedding light on metabolic pathways and cellular functions. Exploring the atomic level provides a profound understanding of the building blocks of life, enabling biologists to unravel the mechanisms underpinning biological processes and diseases.
History Of Atomic Theory
The history of atomic theory is a captivating journey that spans centuries of scientific exploration and intellectual breakthroughs. From initial ideas to gaining scientific shreds of evidence, the contributions of several scientists ensured that a solid theory is established for these indivisible, indestructible elementary particles called atoms, their structure, nature, and other different aspects.
Over time, advancements in technology and further discoveries have continually refined our understanding of atomic structure. Today, modern atomic theory serves as the foundation for modern interdisciplinary science, providing insights into the properties, interactions, and behavior of matter at the atomic and subatomic levels.
-
How understanding ‘Atomic Theory’ can be useful for biologists?
The atomic theory forms a crucial link between the fields of Physics and Biology. The principles of atomic theory directly apply to biological systems enabling us to explore and explain various biological phenomena.
- Atomic theory elucidates the ‘composition of bio-molecules’. Understanding the structure and arrangement of atoms within molecules like proteins, nucleic acids, and carbohydrates is essential for comprehending their functions and interactions in biological processes.

Figure 3: This is the basic structure of a protein and you can see the different types of atoms (oxygen, hydrogen, carbon, nitrogen, etc) that constitute a protein. Image Credit: Western Oregon University. - Atomic theory helps us grasp the ‘principles of molecular biology’, such as enzyme catalysis, molecular structures, and protein folding. The behavior of atoms and their interactions within biomolecules governs the intricate mechanisms that drive enzymatic reactions, ensuring biological reactions occur with precision and efficiency.
- Atomic theory plays a significant role in ‘understanding drug interactions’ within the body. By studying the atomic-level interactions between drugs and their target molecules, scientists can design more effective medications with fewer side effects.
- The study of atomic theory is closely linked to ‘biochemistry and metabolism’. Knowing how atoms combine and rearrange during metabolic processes provides insight into energy production, nutrient utilization, and the synthesis of complex biomolecules.

Figure 4: The infographic shows the importance of a specific atom “CARBON ATOM” in different biochemical pathways. Image Credit: Tim Doheny-Adams. - Last but not least, atomic theory contributes to the ‘field of molecular genetics‘ by explaining the structure and function of DNA. The double helix structure of DNA relies on the complementary pairing of nucleotide bases, which are held together by hydrogen bonds — a concept derived from atomic theory.
-
In philosophy
The idea of the atom being an indivisible particle that constitutes all matter in the world was ruminated in many ancient cultures. Throughout history, various civilizations like Greek, Indian, Chinese, etc pondered the nature of matter.
- In ancient Greece, philosophers like ‘Democritus and Leucippus’ proposed the idea of atoms as the tiny indivisible particles that compose all matter.
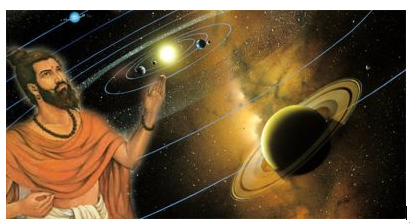
- Similarly, in ancient India, the concept of ‘anu’ emerged in Vedic and Jain literature where ‘Anu’ referred to the smallest particle, indivisible and indestructible, which constituted matter.

Figure 5: An Indian sage named “Kanad” first hinted at the idea of an atom by describing it as the tiniest entity of all matter. He called it “anu”. Image Credit: Vaisheshik Sutra. - In ancient China, the ‘philosophy of Taoism’ introduced the concept of ‘qi’ or ‘chi,’ often associated with the vital energy that permeates all things. The understanding of qi implied the existence of minute, indivisible particles that contribute to the composition of matter.
Although these ancient civilizations lacked the scientific tools to directly observe atoms, their philosophical contemplations laid the groundwork for later scientific investigations.
-
Dalton’s law of multiple proportions
While ancient musings about the atom demonstrate the innate human curiosity and intuition to comprehend the nature of matter even before scientific methods were available to validate such concepts, the development of modern science demanded pieces of evidence.
It was not until the 19th century that John Dalton’s work and subsequent advancements in experimental techniques provided more substantial evidence for the existence of atoms as discrete, indivisible entities. Dalton’s atomic theory gained traction and set the stage for further scientific discoveries regarding atomic structure and behavior.
Dalton was the first person to associate the terminology atom with the discrete units of chemical elements that denote their atomic weight. John Dalton’s major contribution to the scientific world was the discovery of a pattern called the ‘law of multiple proportions’. While at the time, the atom was regarded as the most fundamental unit of matter, it was only after a century that atoms were discovered to be further divisible into protons, neutrons, and electrons (other subatomic particles).
The law of multiple proportions states that the content of different elements constituting a chemical compound varies in weight and this difference depends on some ratios of small whole numbers. In simple words, the law of multiple proportions explains how different chemical elements come together via a ‘basic unit of weight’ which came to be known as an atom.

-
Isomerism
Isomerism is a captivating phenomenon that unlocks the hidden secrets of atoms and finds its profound relevance in the intricate web of biology. At its core, isomerism reveals that ‘molecules with identical atomic compositions can possess strikingly different properties’. For clarity, this can be understood as twins with distinct personalities.
The work of some scientists becomes pivotal when talking about isomerism:
- Friedrich Wöhler (1827): He uncovered that silver fulminate and silver cyanate have the same atomic constitution but very different properties.
- Jöns Jacob Berzelius (1830): He coined the term isomerism.
- Louis Pasteur (1860): He laid the hypothesis of isomerism.
- Jacobus Henricus van ‘t Hoff (1874): He proposed the idea of ‘tetrahedral carbon bonding’ that shed light on organic molecule structures and prediction of the number of putative isomers.

NOTE IT!
“Isomerism in Biological World”
This mind-bending concept of isomerism challenges our conventional understanding of matter, showcasing the incredible versatility of atoms in orchestrating the complexity of life. Via isomerism, atoms can subtly rearrange their atomic arrangement.
Sometimes isomerism can transform some substances ‘from harmless compounds to potent drugs’ or ‘from benign sugars to toxic counterparts’. And this makes the idea of isomerism of atoms an extremely important topic for biologists.
Some examples to understand the isomerism concepts are listed here:
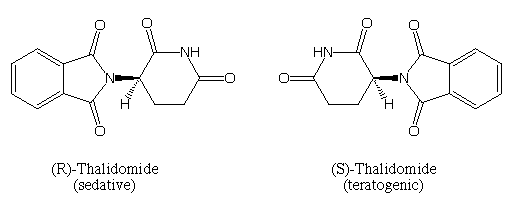
- One interesting case is of thalidomide, a drug once prescribed as a sedative and anti-nausea medication. It was later discovered that ‘one isomer of thalidomide provided the desired effects’, while the ‘other isomer caused severe birth defects’. This stark difference in biological activity highlights the critical role that isomerism plays in drug design and the need for meticulous consideration of the specific isomeric form. You can also listen to this interesting podcast on The Thalidomide Tragedy by Hopewell Valley Student Publications Network where they discuss how the use of incorrect isomer can wreak havoc.https://hvspn.com/captivate-podcast/chem2-7/

Figure 8: While the R-isomer is a sedative, the S-isomer is a teratogenic meaning it can cause rare birth defects in babies (like phocomelia). Image Credit: Pete Gannett. - Another interesting example of isomerism that’s integral in the biological world is the relation of slight variations in isomeric configurations of sugars to significant differences in their taste and metabolic effects. For instance, the isomers of glucose and fructose may have distinct sweetness levels and varying impacts on blood sugar regulation. These subtle changes in structure can influence the body’s response to these sugars, emphasizing the importance of isomerism in understanding their physiological effects.
These profound impacts of the isomerism concepts illustrate their significance in Biology. The ability of isomers to elicit diverse biological responses underscores the significance of studying their structures and properties to ensure the safe and effective development of drugs and the understanding of the intricate mechanisms within living organisms.
-
Brownian motion
This phenomenon was discovered by Robert Brown in 1827 and hence was named after him. Brownian motion is explained as the constant jiggle of dust particles without apparent reason. It was only in 1905 that Albert Einstein theorized this phenomenon and subsequently developed a mathematical model for its scientific description. And finally, it was only in 1908 that Jean Perrin experimentally validated Einstein’s model.
There are several reasons why Brownian motion becomes important from a biological perspective:
In biological systems, Brownian motion influences various processes at the cellular and molecular levels. Examples:
» Movement of molecules within a cell (facilitation of crucial processes such as diffusion, ensuring the distribution of nutrients, signaling molecules, and waste products throughout the cell)
» Molecular interactions (molecular collisions provide the necessary energy for molecular encounters and biochemical reactions and eventually influence reaction rates and the overall dynamics of cellular processes)
» Microbiology (microorganisms dispersal like bacteria or spores enabling them to explore and colonize different habitats)
» Drug delivery and nanomedicine (for optimization of therapeutic outcomes and development of effective drug delivery systems)

-
Discovery of the electron
The discovery of electrons is credited to Sir J. J. Thomson. His experiments with cathode rays elucidated their particular nature meaning particle-like nature instead of wave-like nature.
Some major contributions of J.J. Thomson could be listed as follows:
-
- Discovered cathode rays were made of particles, not waves.
- Determined particles were lighter than hydrogen atoms, indicating their subatomic nature.
- Coined term ‘corpuscles,’ later renamed electrons.
- Linked electrons to particles emitted by photoelectric and radioactive materials.
- Identified electrons as carriers of electric currents in metals.
- Challenged Dalton’s idea of indivisible atoms.

Figure 10: An illustration of J.J. Thomson. Image Credit: Stephen Klassen.
-
Discovery of the nucleus
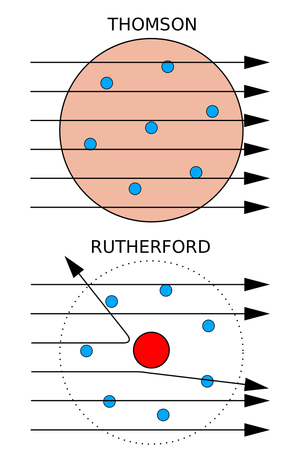
The discovery of the positively charged nucleus revolutionized our understanding of the atomic structure and paved the way for further exploration of subatomic particles. While Rutherford, Geiger, and Marsden encountered scattering issues while trying to measure the charge-to-mass ratio of alpha particles. This led to the discovery of the nucleus while running the Geiger–Marsden experiments. Since alpha particle bombardment was repeatedly yielding deflections at angles greater than 90°, it was proposed that a concentrated positive charge sits at the center of an atom which later came to be known as the ‘nucleus’ of the atom.
Thereafter, Rutherford’s atomic model replaced Thomson’s idea of distributed positive charge throughout the atom.
-
Discovery of isotopes
The discovery of isotopes in 1913 by Frederick Soddy was a monumental event. It was Margaret Todd who introduced the term ‘isotope’ to describe these atoms of the same element with varying weights.
Isotopes are used extensively in the biological world for a variety of purposes.
» For tracing: Isotopes are powerful tools for tracing and studying the movement of substances within living organisms. They are also used as markers to track metabolic reactions, nutrient uptake, and the cycling of elements in ecosystems.
» For ecological studies: Stable isotopes provide valuable information about dietary patterns, migratory routes of animals, and food web dynamics. Isotopic analysis helps biologists understand ecological interactions, population dynamics, and the impact of environmental changes on organisms.
» For archeological and biological dating: Isotopes are used in radiometric dating to determine the age of fossils and the chronology of evolutionary events.
» For medical testing: Isotopes find applications in diagnostic imaging and radiotherapy treatments in medical research, aiding in the detection and treatment of diseases.

-
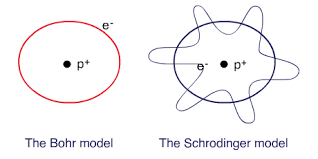
Bohr model
It was in the early 20th century that Niel Bohr came up with a model that changed our understanding of atoms. Imagine an atom as a tiny solar system, with electrons orbiting around a central nucleus, just like planets orbit around the sun. Bohr’s model suggested that these electrons can only exist in specific orbits or energy levels. It’s a bit like saying they can only travel on certain paths around the nucleus. These energy levels are like the floors of a building, where each floor represents a different amount of energy for the electrons.
One of the interesting things about Bohr’s model is that ‘electrons can jump from one energy level to another’, but they can’t be in between. It’s like taking an elevator that only stops on certain floors. When electrons jump between energy levels, they either absorb or emit light energy in the form of tiny particles called photons.
Plus points of Bohr Model:
» This model helped scientists understand why elements produce colorful light when heated or excited. Each element has its own unique set of energy levels, so when electrons move between them, they release specific colors of light. This is why fireworks or neon signs have different colors.

» This model was a big step forward in explaining how atoms work, and it opened the door to further discoveries in quantum mechanics.
Bohr’s model was the first complete physical description of the atom, establishing its structure, bonding, and spectral lines, though it was later replaced by the Schrödinger model.
-
The Schrödinger model
In the early 20th century, scientists like Erwin Schrödinger changed our understanding of atoms with their groundbreaking ideas. Schrödinger proposed a different way to think about electrons, the tiny particles that orbit around the nucleus of an atom.
Instead of seeing electrons as tiny balls, Schrödinger suggested that they behave more like waves. It’s a bit like thinking of them as ripples in a pond. To describe this wave-like behavior, Schrödinger developed an extraordinary equation called the ‘Schrödinger equation’. This equation helps us understand how electrons move and interact with each other.
Schrödinger’s model of the atom introduced the concept of ‘electron waveforms’, which showed that electrons don’t have definite positions like we’re used to in our everyday world. Instead, they exist in what we call ‘probability clouds’ or ‘electron orbitals’ around the nucleus. These orbitals represent the areas where an electron is most likely to be found.
-
Discovery of the neutron
The physicist James Chadwick discovered the neutron in 1932 through a series of experiments involving the bombardment of beryllium with alpha particles. He observed that the radiation produced in this process was not affected by electric fields, indicating the presence of a neutral particle.
The discovery of neutrons helped explain the stability of atomic nuclei. Neutrons, along with protons, form the nucleus of an atom. The neutrons’ presence contributes to the overall mass of the nucleus without introducing additional positive charge.
Neutrons have extensive applications in various scientific fields. Neutron scattering techniques are employed to study the structure and dynamics of materials, including complex biological molecules. Neutron activation analysis is used for elemental analysis in fields such as archaeology, environmental science, and forensic investigations. Neutrons are also utilized in neutron radiography and neutron therapy for medical purposes.

Fission, high-energy physics, and condensed matter
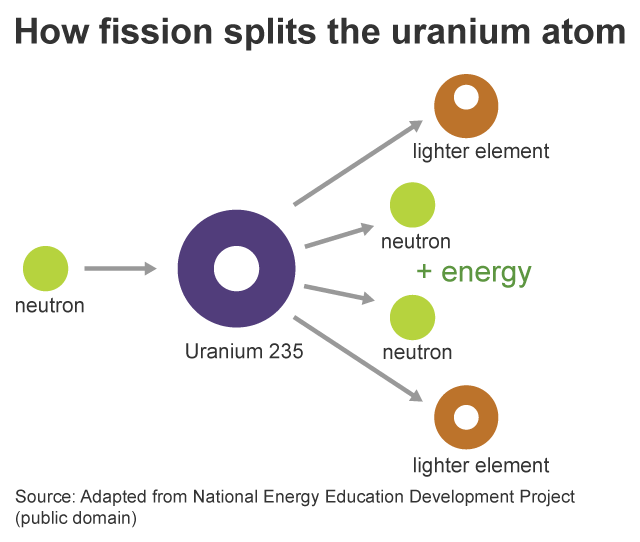
- Fission: Fission is a process where the nucleus of an atom splits into two smaller nuclei, releasing a large amount of energy. The discovery of nuclear fission began in 1938 when Otto Hahn, a German chemist, and student of Rutherford, experimented. He aimed to produce transuranium elements by bombarding uranium atoms with neutrons. Surprisingly, Hahn’s chemical experiments revealed the formation of barium instead. It was Lise Meitner, along with her nephew Otto Frisch, who recognized that Hahn’s unexpected results were the first experimental evidence of nuclear fission. This groundbreaking discovery marked a significant milestone in understanding the behavior of atomic nuclei. This process is helpful in nuclear power plants to split uranium atoms and produce electricity.

Figure 16: A flowchart showing how fission works! Image Credit: EIA. - High-Energy Physics: High-energy physics is the study of particles and strong nuclear force at extremely high energies.
- Condensed Matter: Condensed matter physics is the study of how matter behaves in its solid and liquid forms.
Structure Of Atom
Atomic structure describes how subatomic particles are arranged and organized within an atom. It includes:
» The central nucleus (protons plus neutrons)
» Surrounded by electrons orbiting in specific energy levels.
-
Subatomic particles
Atoms, the building blocks of matter, are composed consist of different subatomic particles. While negatively charged electrons orbit the nucleus, the nucleus itself is composed of protons and neutrons. Protons are positively charged particles (positively charged protons), while neutrons have no charge.
Inside protons and neutrons, there are even smaller particles called quarks (quarks assemble to form protons), held together by strong interactions.
-
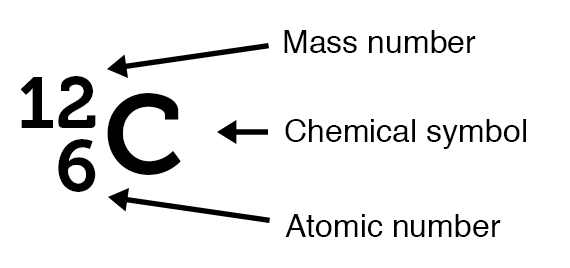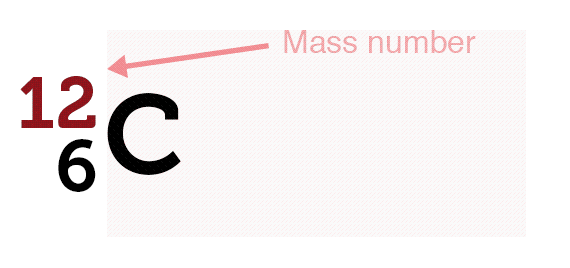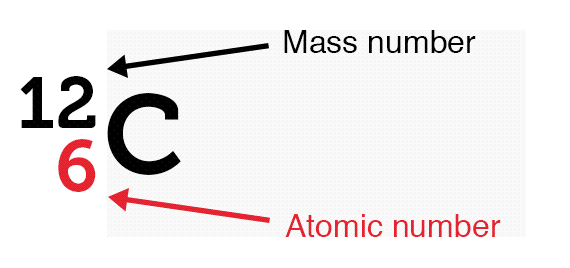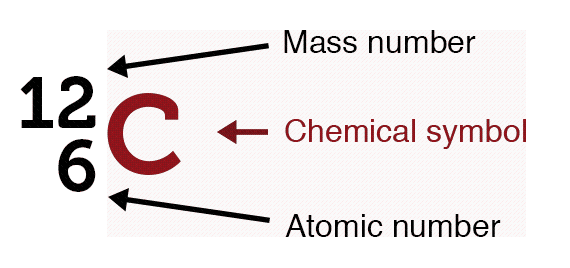
Nucleus
The nucleus of an atom consists of protons and neutrons. They are together referred to as nucleons. All of an element’s atoms possess the same number of protons, known as the atomic number. The only thing that can vary is the number of neutrons leading to the idea of isotopes.
Virtually all the mass of the atom is because of the nucleus and there’s a lot of space. Protons, electrons, and neutrons are fermions and follow the Pauli exclusion principle, meaning no two electrons can occupy the same quantum state.
-
Electron cloud
- Electrons are attracted to protons.
- The force is called electromagnetic force.
- This leads to the creation of ‘an electrostatic potential well’
- Specific energy levels are home to different electrons.
- Standing waves are formed and called atomic orbitals.
- Electrons bound at a distance need less energy to escape than those bound near the potential well.

Properties
Some of the important properties of atoms are discussed here.
-
Nuclear properties
Some of the most important nuclear properties of an atom include:
- Atomic number
- Atomic mass (denoted by atomic mass unit)
- Isotopes
- Radioactivity
- Half-life
- Nuclear binding energy
- Nuclear reactions
- Nuclear stability
-
Mass
Some of the most important mass properties of an atom include:
- Mass number
- Average atomic mass (The mass of an atom at rest is commonly measured in daltons (Da) or unified atomic mass units (u). One dalton is defined as one-twelfth the mass of a free neutral atom of carbon-12. This value is approximately 1.66×10−27 kg.)
- Mass defect
- Binding energy per nucleon
-
Shape and size
Some of the most important shape and size properties of an atom include:
- Atomic radius (the smallest atom is of helium; radius= 32 pm)
- Electron cloud
- Orbitals
- Electron shell
- Molecular geometry
- Bond length
-
Radioactive decay
Some of the most important radioactive decay properties of an atom include:
- Half-life
- Decay mode
- Decay constant
- Radioactive decay chain (Ratio of neutrons and protons determines the stability of the nucleus)
- Radioactive decay products
- Radiation emission
-
Magnetic moment
The magnetic moment of an atom refers to the measure of its magnetic properties. It describes the strength and direction of the magnetic field produced by the atom or its subatomic particles, such as electrons or nuclei. An analogy can be drawn to an object’s angular momentum.

-
Energy levels
Energy levels of an atom refer to the specific quantized states that electrons can occupy within the atom. These levels represent the different energy values at which electrons orbit the nucleus, determining their stability and the amount of energy required for transitions between levels.
-
Valence and bonding behavior
Valence refers to the outermost electron shell of an atom and the number of electrons it can gain, lose, or share during chemical bonding. The bonding behavior of an atom is determined by its valence electrons, which interact with other atoms to form chemical bonds, resulting in the formation of molecules and compounds.
-
States
In the solid state, atoms are closely packed and maintain a fixed position, vibrating about their equilibrium positions. In the liquid state, atoms are less tightly packed and can move more freely, sliding past each other. In the gas state, atoms are widely spaced and move independently, with high kinetic energy and rapid random motion.
Identification
Several identification methods are used to visualize and analyze atoms in various contexts. These methods aid in determining atomic composition, isotopic ratios, chemical species, and structural properties, enabling scientific research, material characterization, and understanding of atomic behavior in different environments.
Some of the most frequently used ones are:
- Scanning tunneling microscope (STM)
- Mass spectrometer
- Inductively coupled plasma atomic emission spectroscopy
- Inductively coupled plasma mass spectrometry
- Atom-probe tomograph
- X-ray photoelectron spectroscopy (XPS)
- Auger electron spectroscopy (AES)
- Electron energy loss spectroscopy (EELS)
- Analysis of spectra of excited states in distant stars
- Gas-discharge lamp spectroscopy
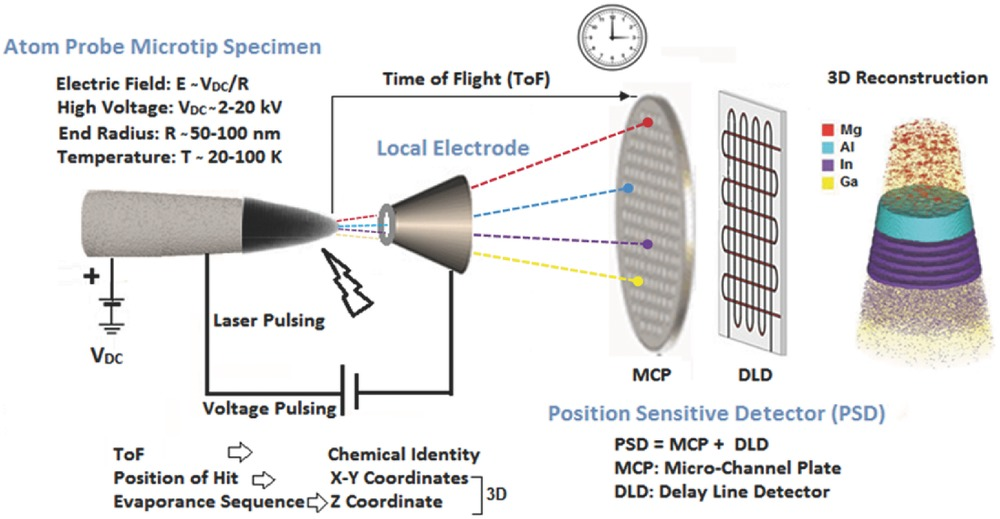
These identification methods are highly useful in biology as they have consistently demonstrated their significance in advancing our understanding of biological systems, human health, and the development of novel therapeutics and diagnostics. Some of the main biological domains that have benefitted from these methods are:
- Protein structure determination
- Drug discovery
- Molecular modeling
- Metabolomics
- Biomolecular interactions
- Structural biology
- Cell imaging
- Genomics and sequencing
- Forensic analysis
- Disease diagnosis and biomarker discovery
Origin And Current State
Atoms, the fundamental building blocks of the universe, have a fascinating origin and exist in different states today. In the vast observable universe, atoms account for approximately 4% of the total energy density. Their average density is around 0.25 particles per cubic meter, mainly consisting of protons and electrons. Within our galaxy, the Milky Way, atoms are more concentrated. In the interstellar medium, the spaces between stars, the density can range from 100,000 to 1 billion atoms per cubic meter. However, in our solar neighborhood, the density is much lower, around 1,000 atoms per cubic meter.
-
Formation
In the early stages of the Big Bang, electrons and atomic nuclei have emerged. Subsequently, Big Bang produced helium, lithium, and deuterium. The stability of atoms is attributed to their binding energy, and the prevalence of atoms increased during a phase called recombination when electrons became attached to nuclei. Stars played a crucial role in the synthesis of heavier elements through nuclear fusion, while cosmic ray spallation contributed to the generation of isotopes like lithium-6. Supernovae and colliding neutron stars were responsible for the formation of elements heavier than iron through processes called the r-process and s-process. Lead, for instance, originated from the radioactive decay of heavier elements.
-
Earth
The majority of atoms on Earth formed during the Solar System‘s inception. They provide insights into the Earth’s age through radiometric dating. Helium in the Earth’s crust results mainly from alpha decay. Some atoms are not primordial, including carbon-14 generated by cosmic rays. Artificially created atoms stem from human activities and nuclear reactions. Only plutonium and neptunium occur naturally among transuranic elements, with their quantities diminishing due to radioactive decay. Atoms combine to form compounds like water and salt, while materials like crystals and metals lack molecular interruptions.
-
Rare and theoretical forms
Some rare forms of matter and atoms can only be theoretical or very sparse in the real world.
Superheavy elements
Superheavy elements are synthetic and do not occur naturally. They are created through nuclear reactions in laboratories. These elements have extremely short lifetimes due to their high instability, making them challenging to study. Scientists continue to explore and synthesize new superheavy elements to expand our understanding of the atomic structure and the limits of the periodic table.

Exotic matter
Exotic matter refers to hypothetical types of matter that possess unusual properties not found in ordinary matter. Examples include negative mass, which would exhibit repulsive gravity, and exotic particles like dark matter. While the exotic matter is currently theoretical, its study is important for understanding the fundamental nature of the universe and exploring concepts like warp drives and wormholes.
Take the Atom – Biology Quiz!
Further Reading
References
- Khan, M. A., Ringer, S. P., & Zheng, R. (2016). Atom probe tomography on semiconductor devices. Advanced Materials Interfaces, 3(12), 1500713.
- Perrin (1909). Brownian Movement and Molecular Reality, p. 50
- Zeghbroeck, Bart J. Van (1998). “Energy levels”. Shippensburg University.
- Martin, W.C.; Wiese, W.L. (May 2007). “Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas”. National Institute of Standards and Technology.
- Pullman (1998). The Atom in the History of Human Thought, p. 198: “Dalton reaffirmed that atoms are indivisible and indestructible and are the ultimate constituents of matter.”
- Reusch, William (16 July 2007). “Virtual Textbook of Organic Chemistry”. Michigan State University.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.