Molecule
n., plural: molecules
[ˈmɒl.ɪˌkjuːl]
Definition: A group of atoms bonded together
Table of Contents
Molecule Definition
Two or more atoms (same or different) that are held together by attractive forces or chemical bonds form a molecule. The smallest unit of chemical elements having discrete chemical properties is the atom however, the characteristic of a matter depends upon the bonding between the atoms. Thus, a molecule forms the smallest unit of a matter that has distinctive physical and chemical properties of a matter.
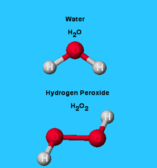
The simplest example of a molecule is a water molecule formed by two atoms of hydrogen atoms and one oxygen atom and is represented by the chemical formula H2O (Figure 1).
There are 92 naturally found elements however, living beings are primarily composed of four elements Hydrogen (H2), Carbon (C), Oxygen (O2), and Nitrogen (N2).
Most of the molecules are very small and not visible to the naked eye. The size of molecules is measured in Angstrom (Aº).
- The smallest molecule is hydrogen (H2) wherein two hydrogen atoms combine to form a hydrogen molecule.
- Diatomic molecules are those wherein only two atoms combine to form a molecule.
- Hydrogen (H2), Oxygen (O2), Nitrogen (N2) are all examples of homonuclear diatomic molecules, as same type of two atoms combined to form a molecule.
- CO2 (one carbon atom bonds with two oxygen atoms) and H2O are an example of heteronuclear diatomic molecules as two different types of atoms bond to form a molecule.
Video: What is a molecule?
Biology definition:
A molecule is the smallest unit of an element or compound, made up of two or more atoms held together by a strong chemical bond, which can be covalent, where electrons are shared, or ionic, where electrons are transferred.. The atoms comprising the molecule may be of the same kind (as in an oxygen molecule made up of two oxygen atoms) or of different kinds (such as a water molecule made up of oxygen and hydrogen). A molecule has distinct physical and chemical properties of a substance.
At the cellular or biochemical level, a molecule refers to any minute particle such as charged organic molecules, or to substances (called biomolecules) produced and occur naturally in living organisms such as proteins, carbohydrates, DNA, etc. Molecules can vary greatly in size and complexity, from simple diatomic molecules like oxygen (O2) to complex organic molecules such as DNA.
Etymology: The word “molecule” derives from the Latin “molecula,” which means a small mass or particle.
Synonym: atom; particle; compound; entity
Compare: element, ion
See also: biomolecule
Characteristics Of Molecules
The basic characteristics of a molecule are:
- A molecule is the smallest unit of matter that has distinctive chemical and physical properties.
- A molecule owns a definite size and mass.
- Two or more atoms (same or different) held together by a chemical bond form a molecule wherein the ratio of two atoms is fixed in an element.
- The property of a substance/matter is lost when a molecule breaks. The breakdown of a molecule results in individual atoms that have different chemical and physical properties than the molecule.
NOTE IT!
Molecules can acquire distinct shapes or molecular structures like, linear, V-shaped, trigonal planar, pyramidal, trigonal pyramidal, tetrahedral, octahedral, and T-shaped.
Molecular Bonding
Briefly, an atom has positively charged particles (protons) in the nucleus along with neutral particles (neutrons), while, negatively charged particles (electrons) in equal numbers to protons are present around the nucleus in an electronic cloud or valence shells or orbits.
An equal number of protons (positively charged particles) and electrons (negatively charged particles) impart a neutral charge to an atom. The number of protons in the nucleus forms the atomic number of an atom while the sum of the number of protons and neutrons forms the atomic mass or mass number of the atom.
The protons and neutrons are tightly bound in the nucleus and only under extreme conditions do the protons and neutrons separate out (as seen in radioactivity). However, the electrons revolving around the nucleus are present in the orbits or shells that are bound by the electrostatic forces.
The electrons in the outermost shell are involved in chemical bond formation, which determines an atom’s reactivity:
- The number of electrons in each orbit is strictly fixed. The innermost orbit electrons experience strong electrostatic force while the outermost orbit electrons experience weak electrostatic forces. Thus, the electrons in the outermost orbit are available for sharing with other atoms, i.e., chemical bonds with the other atoms.
- The number of electrons in the outermost orbit is referred to as valence electrons. Accordingly, the number of electrons determines the chemical property of the atom and all the atoms of an element have the same number of electrons.
- The number of electrons that are involved in the chemical bond formation or sharing with the other atom determines the activity of the atom. An atom can form a single bond, double bond, or triple bond by sharing one, two, or three electrons, respectively.
-
- The bond formed by the sharing of electrons between two atoms is referred to as a covalent bond. Covalent bonds are strong bonds that are capable of storing energy and hence biological molecules are formed by covalent bonding so that living beings use this energy for their survival and growth. Thus, to harness the energy of living beings these covalent bonds need to be broken. Living beings use enzymes to break down these covalent bonds and derive energy from them.
- The bond formed due to electrostatic attractive forces between two oppositely charged atoms is referred to as an ionic bond that results in the formation of ionic compounds. Thus, the atom that loses one or more electrons acquires a net positive charge and is referred to as a cation (sodium ion, Na+) while the atom that gains one or more electrons acquires a net negative charge and is referred to as an anion (chloride ion, Cl–). Due to electrostatic attractive forces between positively charged Na+ (cation) and negatively charged Cl– (anion), ionic compound NaCl, is formed.
At room temperature and pressure, the ionic compounds are usually solid or sometimes liquids.
Examples Of Molecule
-
Carbon-Based Molecules
Carbon is one of the essential elements that form the basis of living beings. Carbon has a valency of 4 thus it has the ability to form four covalent bonds with other atoms. Thus, in living beings, carbon forms several molecules of different sizes (both long and short molecules) in association with H2, O2, and N2. According to various evolutionary theories, the early evolution of life on Earth took place due to the formation of various molecules in association with carbon. A double bond between two carbon bonds is flexible and imparts rotatability to the molecule. Thus, this flexibility of the molecule helps the molecule to interact with a large number of other molecules like binding at the receptor.
-
Adenosine Triphosphate (ATP)
Almost all living beings use Adenosine Triphosphate (ATP) molecules to harness the energy required to carry out all living processes. ATP molecules are produced in the body during the process of respiration and glycolysis (i.e., the breakdown of the glucose molecule).
ATP molecule is a complex big molecule composed of carbon, oxygen, phosphorous, and nitrogen. ATP molecule has a cyclic structure with a phosphate group (PO4–) at one end. Breakage of covalent bonds in the phosphate group of the ATP molecule yields energy. An ATP molecule functions as a coenzyme and energy generated due to the breakage of the phosphate bond is transferred to the enzyme involved. Further, the energized enzyme accelerates the biochemical processes. As a result of the breakdown of the phosphate group from the ATP molecule results in the formation of two molecules, a free phosphate group, and an adenosine diphosphate (ADP) molecule.
Types Of Biological Molecules
The majority of biological molecules are formed by the combination of carbon, oxygen, nitrogen, and phosphorous. The molecules formed may be small like H2O or complex polymeric molecules like carbohydrates, proteins, and fatty acids.
-
Proteins
Proteins are the building blocks of all life forms on Earth. Proteins are polymeric molecules that are formed by the bonding between several monomeric units or molecules. In proteins, amino acids combine to form the final complex polymeric protein structure. The DNA of each cell encodes amino acids. Amino acids combine in a specific sequence to form a peptide structure and eventually a complex folded protein molecule. Proteins are used for a variety of functions, like, enzymes that catalyse various biochemical processes are protein molecules. Hormones required for the metabolic and growth processes are also proteinaceous molecules. Antibodies that protect the body from pathogens are also made up of proteins. Proteins are also used for building the structural component of the body. There are various other functions of proteins in the body.

-
Lipids
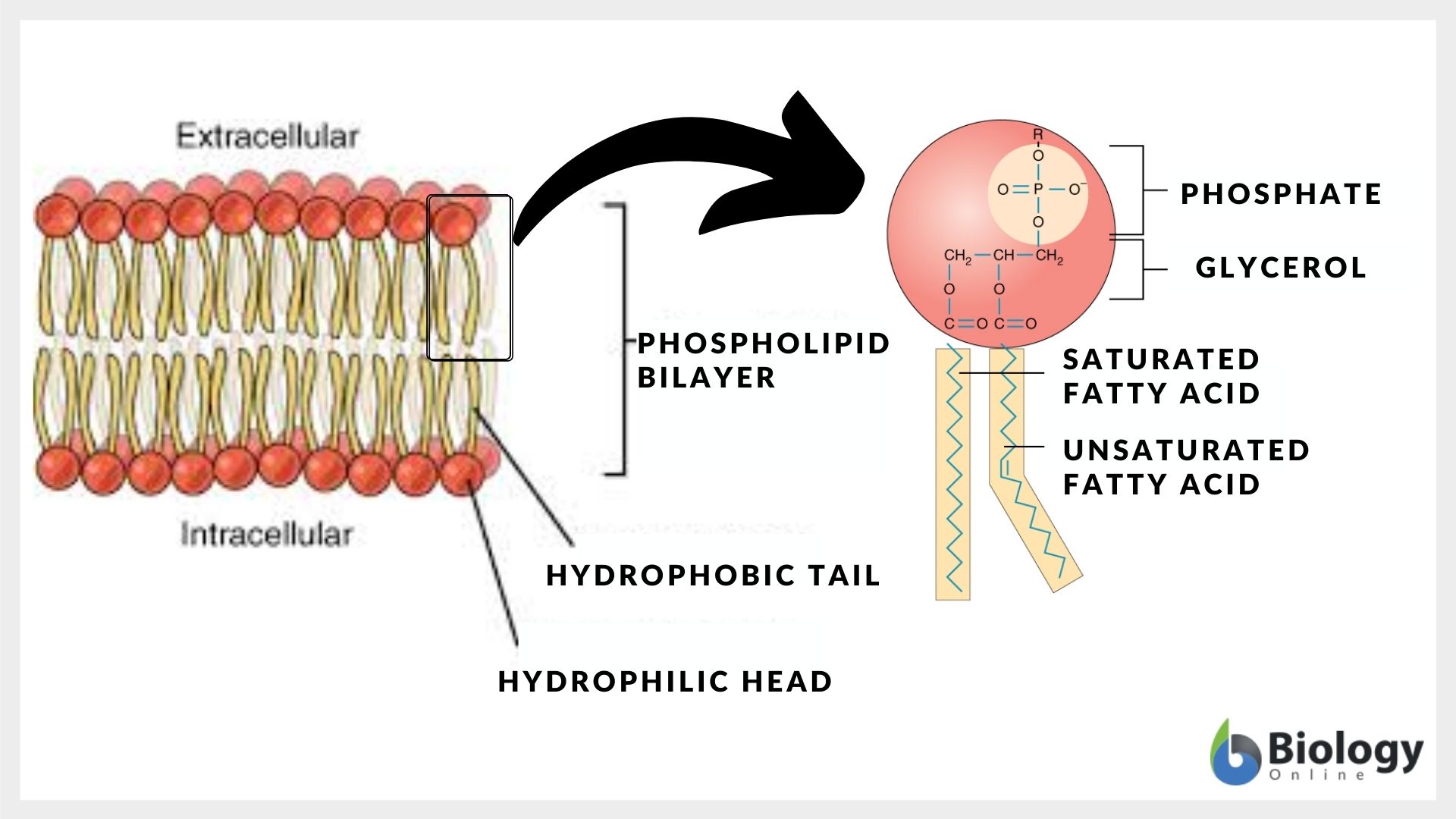
Lipids are organic compounds that encompass fats, oils, glycerides (di-/tri), waxes, steroids, and phospholipids. Lipids are the ester of the glycerol and fatty acid molecules. Lipid molecules are of various lengths and different degrees of saturation that end with the free carboxylic acid group. Lipids are water-insoluble and soluble in non-polar. Thus, lipids are non-polar molecules.
A phospholipid molecule is, however, amphiphilic in nature (i.e., it has both water-loving and oil-loving characteristics). In phospholipid molecules, the phosphate group is attached to the lipid molecule hence, the phosphate part of the molecule attracts water molecules while the lipidic part of the molecule attracts lipid/oil molecules. Thus, having an amphiphilic nature. Phospholipid is the essential component of all cell membranes.
-
Carbohydrates
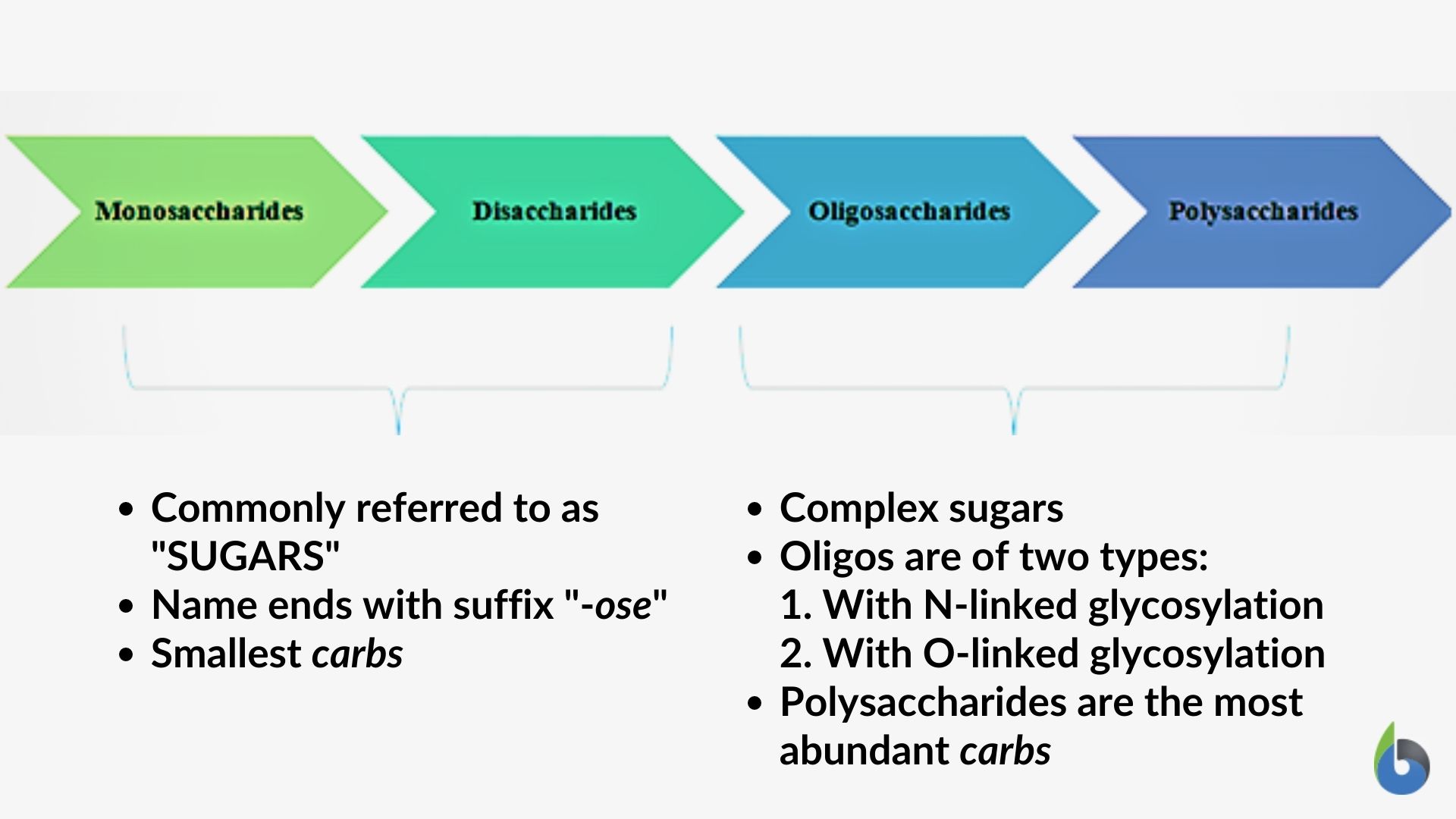
Carbohydrates are referred to as energy-providing molecules in the body. Animals use glucose molecules for deriving energy while plants generate complex carbohydrates like starch. Carbohydrates are made up of monomeric units referred to as saccharides that combine to form polysaccharides. Plants use complex carbohydrates- cellulose for structural function as well. Cellulose is an important component of the plant cell wall. Plants, with the help of light energy, produce glucose molecules and energy is stored in the glucose molecule. The glucose molecules further polymerize to form polysaccharides. The animals feed upon the plants and during the process of digestion break down polysaccharide molecules into glucose. The glucose is then utilized by the animals to generate ATP molecules which in turn provides energy required for the life processes.
-
Nucleic Acids
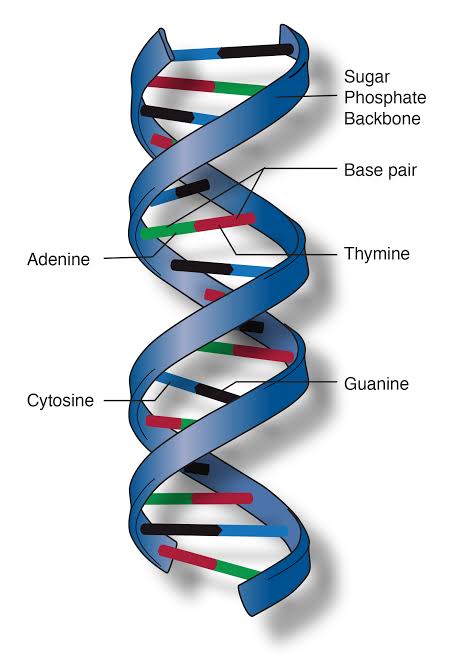
Nucleic acids are the basis of all life processes. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are composed of a sequence of nucleic acid. All the life processes are encoded in the nucleic acid of a cell. In a DNA molecule, nucleic acids are the nitrogenous organic compounds namely, adenine (A), thymine (T), guanine (G), and cytosine (C).
In DNA, various permutations and combinations of A, T, G, and C encode all the essential information required for life processes. The various combinations of A, T, G, and C codes for amino acids which are eventually converted to proteins. All the genetic information is passed from one generation to another through nucleic acids in the DNA/RNA.
Take the Molecule – Biology Quiz!
References
- Bader, R. F. (1985). Atoms in molecules. Accounts of chemical research, 18(1), 9-15.
- · Bao, G. (2002). Mechanics of biomolecules. Journal of the Mechanics and Physics of Solids, 50(11), 2237-2274.
- · Edwards, C., Lai, T., Ros, K., Honke, G., Cho, K., & Ji, H. (2022). Translation between molecules and natural language. arXiv preprint arXiv:2204.11817.
- · Edwards, C., Lai, T., Ros, K., Honke, G., Cho, K., & Ji, H. (2022). Translation between molecules and natural language. arXiv preprint arXiv:2204.11817.
- · Li, Y., & Zhao, D. (2013). Basics of Molecular Biology. Molecular Imaging: Fundamentals and Applications, 541–601. https://doi.org/10.1007/978-3-642-34303-2_16.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.