Search Results for: bond
Covalent bond
Covalent Bond Definition What is a covalent bond? In chemistry and other fundamental science fields, a covalent bond is... Read More
Hydrogen bond
Definition noun plural: hydrogen bonds A type of chemical bond that is formed when the slightly positive hydrogen atom of... Read More
Ionic bond
Definition noun plural: ionic bonds A type of chemical bond in which atoms, ions, or molecules are held together by... Read More
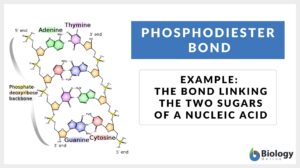
Phosphodiester bond
Phosphodiester Bond Definition Phosphodiester bonds are the backbone of the strands of nucleic acid present in the life... Read More
Chemical bond
Definition noun, plural: chemical bonds The attractive force that binds atoms, ions, or molecules in a chemical... Read More
Isopeptide bond
Definition noun, plural: isopeptide bonds A peptide bond formed between a carboxyl group of one amino acid and an amino... Read More
Peptide bond
Definition noun, plural: peptide bonds (1) The covalent bond joining amino acids, particularly at the carboxyl group of... Read More
Disulfide bond
Definition noun (chemistry) (1) The single covalent bond formed from the coupling of thiol groups, especially of cysteine... Read More
Coordinate covalent bond
Coordinate covalent bond --> semipolar bond a bond in which the two electrons shared by a pair of atoms belonged originally... Read More
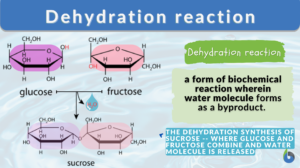
Dehydration reaction
What is dehydration synthesis? A dehydration reaction is a form of biochemical reaction wherein a water molecule is lost or... Read More
Chemical Composition of the Body
In order to fully understand the mechanisms of human physiology, it is important to have an understanding of the chemical... Read More
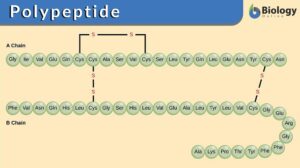
Polypeptide
Polypeptide Definition Biology What are polypeptides? A polypeptide is defined as a polymer of amino acids joined together... Read More
Double bond
Double bond (Science: chemistry) a covalent bond resulting from the sharing of two pairs of electrons; e.g., H2C==CH2... Read More
Single bond
single bond A covalent bond resulting from the sharing of one pair of electrons; e.g., H3C-CH3... Read More
Glycosidic bond
Glycosidic bond is a covalent bond that holds a carbohydrate (sugar) to another group that can or cannot be another... Read More
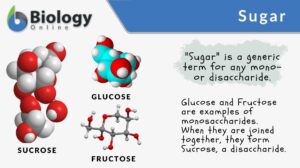
Saccharide
Saccharide Definition What is a saccharide molecule? A saccharide is the unit structure of carbohydrates. In biochemistry,... Read More
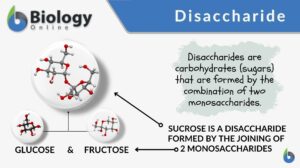
Disaccharide
Carbohydrates are organic compounds comprised of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1. They are one... Read More
High Energy Bond
A type of bond in a molecule that is in a high energy state, such as atp compared to adp, atp has much more energy... Read More
Electrostatic Bond
A chemical bond in which one atom loses an electron to form a positive ion and the other atom gains to electron to form a... Read More
Isomaltose
Definition noun plural: isomaltoses i·so·mal·tose, aɪsoʊˈmɔːltəʊz A disaccharide formed from the combination of... Read More
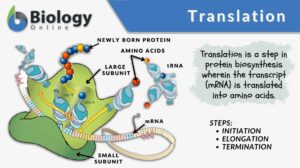
Translation
Translation, in general, is the conversion of something into another form, such as a word from one language to another. But... Read More
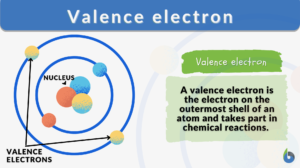
Valence electron
What are valence electrons? Why are they significant? Valence electrons definition in chemistry: The electrons in an atom's... Read More
Isomaltulose
Definition noun plural: isomaltuloses A disaccharide comprised of a glucose monomer and a fructose monomer joined by... Read More
Cellobiose
Definition noun plural: cellobioses cel·lo·bi·ose, ˌsɛləʊˈbaɪəʊz A disaccharide made up of two glucose... Read More
Polyunsaturated fatty acid
Definition noun, plural: polyunsaturated fatty acids Any of a group of unsaturated fatty acids characterized by having more... Read More
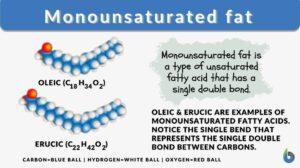
Monounsaturated fat
What is monounsaturated fat? Monounsaturated fats are healthy dietary fats. They are liquid at room temperature. Unlike... Read More
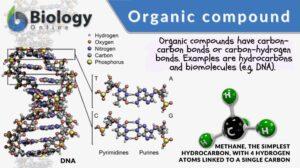
Organic compound
Organic Compound Definition An organic compound is a compound that, in general, contains carbon covalently bound to other... Read More