Oxygen
n., plural: oxygens
[ˈɒksɪdʒən̩]
Definition: the colorless, odorless, gaseous element represented by the symbol “O”, with an atomic number of 8
Table of Contents
Oxygen Definition
In biochemistry, oxygen is the colorless, odorless, gaseous element represented by the symbol “O”, with an atomic number of 8, and constitutes about 21% by volume of the atmosphere, and biologically important for its role in various biochemical and physiological processes, especially of aerobic organisms. Etymology: Ancient Greek ὀξύς (oxús, meaning “sharp”) + γενής (-genēs, meaning “producer”). Symbol: O.
Oxygen is one of the chemical elements found in nature. A chemical element refers to the pure substance of one type of atom. At present, 94 are natural elements whereas 24 are synthetic. Oxygen is one of the most common elements in living things, together with carbon, hydrogen, and nitrogen. It is also the third most-abundant element in the universe, next to hydrogen and helium.
Properties of Oxygen
Oxygen is a natural gaseous element with an atomic number of 8 and an atomic weight of 15.96. In the periodic table, it belongs to the chalcogens. It is a reactive nonmetal with an electron configuration of He 2s2 2p4. It is capable of combining with all elements, with the exception of fluorine, to form oxides, bases, oxyacid anhydrides, etc. At room temperature, oxygen is only moderately active with most substances. However, at higher temperatures, it becomes very active that it is considered as one of the most powerful chemical agents. The melting point of oxygen is -218.79 °C. Its density at STP is 1.49 g/L at 0°C and 760 mm·pressure.
In the 18th and 19th centuries, scientists learned that the air components could be liquefied by compressing and cooling the air. In 1883, oxygen was liquefied in a stable state for the first time.1 The liquid oxygen is pale blue in color, the density of 1.141 g/cm3, the boiling point of −182.96 °C at 101.325 kPa (760 mmHg), and freezing point of −218.79 °C. Presently, it is used in military aircraft and gas industries.
Solid oxygen is another physical state of oxygen that forms at normal atmospheric pressure at a temperature below −218.79 °C. It also has a pale blue color. It has a density of 21 cm3/mol in the α-phase to 23.5 cm3/mol in the γ-phase.2
Isotopes of Oxygen
The naturally-occurring isotopes of oxygen are Oxygen-16, Oxygen-17, and Oxygen-18. All three isotopes are stable. Oxygen-16 (16O) has 8 neutrons and 8 protons in its nucleus. It is the most abundant oxygen isotope and accounts for 99.762% of natural abundance (NA, i.e. the abundance of the isotope in nature). Oxygen-17 (17O) has 9 neutrons and 8 protons in its nucleus. Its NA is 0.0373% in seawater and 0.0377421% in seawater. Oxygen-18 (18O) has 10 neutrons and 8 protons in its nucleus. Its NA is 0.2%.
Allotropes of Oxygen
An allotrope of an element pertains to any of the multiple substances formed by only one type of element. Examples of allotropes of oxygen are atomic oxygen, dioxygen, ozone, and tetraoxygen. Atomic oxygen (O1) is a very reactive allotrope of oxygen. It tends to quickly bond with nearby molecules. Dioxygen (O2) (free oxygen) occurs in two major forms: triplet and singlet. Triplet oxygen 3O2 is the triplet ground state of dioxygen. It is better known as molecular oxygen.
Its two oxygen atoms are attached by one full σ bond plus two π half-bonds. It is the most common and the most stable allotrope of oxygen on Earth. This is the form that is utilized by organisms, e.g. in cellular respiration. It is also released as a byproduct of photosynthesis by photoautotrophs.
Singlet oxygen 1O2 is dioxygen with a formula of O=O. It is more reactive to organic compounds than triplet oxygen is. It can be distinguished from triplet oxygen based on the number of electron spins. Singlet oxygen has only one possible arrangement of electron spin whereas triplet oxygen has three. Singlet oxygen is one of the reactive oxygen species (ROS).
In photoautotrophs, singlet oxygen is produced by chlorophyll molecules during photosynthesis. Plants counter the damaging oxidative effect through the action of carotenoids. Herbivores that ingest plant parts rich in chlorophyll pigments that produce singlet oxygen are prone to photosensitivity.
Humans, for instance, that stick to a vegan diet may become more sensitive to light and become predisposed to photodermatitis. In mammals, ROS is associated with the oxidation of LDL cholesterol, which in turn, accounts for the deleterious effects on the cardiovascular system. In medicine, it is the active oxygen species in photodynamic therapy.
Ozone (O3) is a molecule present in the ozone layer of the stratosphere. It is capable of absorbing most of the ultraviolet radiation from the Sun. Tetraoxygen (O4) was also called oxozone.
Oxygenic Compounds
Water (H2O) is one of the oxides of hydrogen and the most common oxide. The hydrogen atoms are bound to oxygen by covalent bonds. Water is a polar molecule due to its oxygen that has a slight negative charge while its hydrogens have a slight positive charge. The polarity of water makes it an excellent solvent. The slightly negative oxygen attracts cations whereas the slightly positive hydrogen attracts anions. Thus, water has the ability to dissociate and ionize molecules. Water, CO2, MgO, Al2O3, Na2O, CaO, BaO, and ZnO are examples of oxides, which are also examples of inorganic compounds containing oxygen.
Organic compounds are fundamentally defined as those substances containing carbon atoms and Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H) bonds. Examples of common organic compounds containing oxygen and R (the organic functional group) are alcohols (R-OH), aldehydes (R-CO-H), amides R-C(O)-NR2, esters (R-COO-R), ethers (R-O-R), and ketones (R-CO-R). Other important organic compounds that have oxygen are citric acid, formaldehyde, glycerol, acetamide, formaldehyde, and glutaraldehyde.
Discovery of Oxygen
In the 17th and 18th centuries, the early experiments by scientists such as Robert Hooke, Ole Borch, and Pierre Bayen led to the production of oxygen. However, it was not recognized as a chemical element back then. Rather, the predominant thought for many centuries was that the four major elements were air, fire, water, and earth. It was not yet known that each of them was comprised of simpler constituents, which later were called chemical elements.
Joseph Priestley 1733 – 1804, the British clergyman, contended this belief and claimed that the air was comprised of substances such as the gas that he observed to have been liberated from mercuric oxide (HgO) in his experiments. He referred to this gas as dephlogisticated air. Later, the gas was given the name oxygène in 1777 by Antoine Lavoisier 1743 – 1794, French chemist.3 Priestley was the first to publish about oxygen and as such was usually ascribed as the discoverer of oxygen.
The English name oxygen was adopted from Lavoisier’s oxygène, which in turn was derived from the Greek ὀξύς (oxús, meaning “sharp”) and -γενής (-genēs, meaning “producer”). It was a misnomer, though, because the element was thought to be a constituent in the formation of all acids. The name was well established that it remained till now even after it was found out not to be true.
Biological Importance
In biology, oxygen plays a crucial role in various biochemical and physiological processes. It is the most abundant element (65% by mass) in the human body, followed by: carbon (18.5%), hydrogen (9.5%), nitrogen (3.2%), calcium (1.5%), and phosphorus (1%).
Respiration
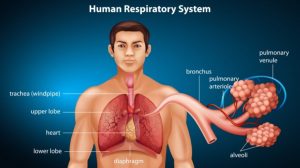
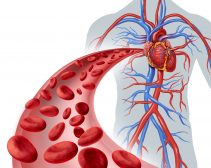
In humans and other terrestrial vertebrates, dioxygen (O2) enters the body through the lungs, and then binds to the hemoglobin of the red blood cells to be delivered to various parts of the body. The dioxygen detaches from the hemoglobin and enters tissues by diffusion. In turn, carbon dioxide is picked up to be brought to the lungs to be released outside.
Oxygen enters the cell to be used by mitochondria to generate ATP through cellular respiration. It acts as the final electron acceptor in the electron transport chain during oxidative phosphorylation. The overall reaction of cellular respiration is: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + 2880 kJ/mol.
Since it utilizes oxygen, the process is described as aerobic. The presence of oxygen makes cellular respiration about ten times more efficient in yielding ATP.
Immune function
In humans, hydrogen peroxide (H2O2), singlet oxygen, and superoxide ions are some of the ROS that naturally occurs as by-products of oxygen use. They are used to destroy pathogens, and therefore have an immune function.
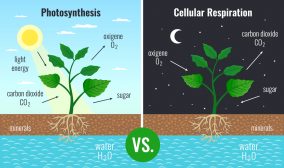
Photosynthesis
Photoautotrophs, such as cyanobacteria, green algae, and plants, produce oxygen through photosynthesis. The overall formula of the process is:
6 CO2 + 6 H2O + photons → C6H12O6 + 6 O2
Carbon dioxide, water, and photons are required to produce glucose and O2. The oxygen is eventually released into the atmosphere.
Oxygen therapy
Oxygen is also thought to have a therapeutic role especially in treating or managing ischemic tissues. Oxygen therapy, the use of oxygen for medical treatment, is used to treat conditions with impaired oxygen uptake, such as pneumonia and emphysema. Oxygen (O2) can be toxic at high partial pressures (<50 kilopascals) though. It can lead to health problems and convulsions.
Geologic History of Oxygen
3.85 to 2.45 billion years ago, there was no free oxygen yet in the Earth’s atmosphere and most oceanic parts were anoxic. Free oxygen began to exist in the atmosphere when photosynthetic organisms evolved. This is believed to have occurred in about 3.5 billion years ago. Through photosynthesis, they utilized carbon dioxide, water, and photons to yield sugars. The oxygen produced from photosynthesis as well was discarded as a waste product.
In 2.45 to 1.85 million years ago, oxygen level started to significantly rise. Much of the free oxygen produced by organisms was absorbed in oceans and seabed rock. The biologically-induced oxygen buildup has been referred to as the Great Oxygenation Event. It is surmised to have occurred during the Siderian period (2.5-2.3 billion years ago) of the Paleoproterozoic Era. The significant buildup of free oxygen caused the extinction of many obligate anaerobes.
Free oxygen began to outgas from the oceans 1.85 to 0.85 billion years ago. The land surfaces absorbed much of it. From then on to the present, free oxygen eventually accumulated in the atmosphere, especially when oxygen reservoirs were filled. The evolution of organisms that could metabolize oxygen curbed the increment of available free oxygen.
In the Carboniferous period (358.9–298.9 million years ago) of the Paleozoic era, the oxygen level in the atmosphere rose to 35% by volume. This was thought to have been a factor in the evolution of large-sized insects and amphibians. It was also hypothesized that the availability of oxygen led to the diversity of aerobic organisms.4
Oxygen Cycle
Oxygen is the third most abundant element in the universe, after hydrogen and helium. It, therefore, widely occurs and is cycled on Earth. Oxygen cycle is one of the biogeochemical cycles on Earth, being converted from one form to another.
The four main reservoirs of oxygen are the atmosphere, hydrosphere, lithosphere, and biosphere. The lithosphere is the largest reservoir, particularly within the silicate and oxide minerals in the Earth’s crust and mantle. In the atmosphere, oxygen occurs predominantly as dioxygen. It also has other oxygenic molecules, such as ozone (O3), CO2, H2O (as water vapor), and other oxides. The high concentration of ozone accounts for the formation of the UV shield, called the ozone layer, in the stratosphere. In the hydrosphere, oxygen occurs in water molecules, in carbonic acids, and as free oxygen. A major source of oxygen is from the biosphere as the byproduct of the biologic process, photosynthesis. Photolysis also forms oxygen. It breaks down water and nitrous oxide to release free oxygen into the atmosphere while hydrogen and nitrogen, into space. Marine animals with calcium carbonate shells also serve as a biological source. When they die, the calcium carbonate in their shell becomes incorporated into the limestone sedimentary rocks.
Free oxygen from the atmosphere is metabolized by aerobic animals for respiration. And in so doing, they release carbon dioxide.
The lithosphere absorbs free oxygen from the atmosphere in chemical weathering, such as in the formation of rust.
Read:
References:
- Papanelopoulou, F. (2013). “Louis Paul Cailletet: The liquefaction of oxygen and the emergence of low-temperature research”. Notes and Records of the Royal Society of London. 67 (4): 355–73. doi:10.1098/rsnr.2013.0047
- Roder, H. M. (1978). “The molar volume (density) of solid oxygen in equilibrium with vapor”. Journal of Physical and Chemical Reference Data. 7 (3): 949. doi:10.1063/1.555582
- Joseph Priestley, Discoverer of Oxygen National Historic Chemical Landmark – American Chemical Society. (2015, January 1). Retrieved from www.acs.org/content/acs/en/education/whatischemistry/landmarks/josephpriestleyoxygen.html
- Hickey, H. (2015, January 1). Oxygen provided breath of life that allowed animals to evolve. Retrieved from www.washington.edu/news/2015/12/18/oxygen-provided-breath-of-life-that-allowed-animals-to-evolve/
© Biology Online. Content provided and moderated by Biology Online Editors