Carbon dioxide
n.
[daɪˈɒksaɪd]
Definition: An inorganic compound composed of two oxygens and a carbon
Table of Contents
Carbon Dioxide Definition
noun, car·bon di·ox·ide, /daɪˈɒksaɪd/ (biochemistry) An inorganic compound, with the chemical formula, CO2, composed of two oxygen atoms linked to a single carbon atom by a covalent bond, and essential to many biochemical and biological processes. Synonyms: carbon oxide, carbon(iv) oxidem carbonic anhydride, carbonic oxide, carbonic-acid gas, dry ice (solid phase).
More About Carbon Dioxide
Carbon dioxide (CO2) became the first gas that was distinguished from ordinary air. In 1630 or so, Jan Baptist van Helmont, a Flemish chemist, identified a vapor distinct from the air when he burned charcoal in a closed vessel. He, them, called the vapor “wood gas” (spiritus sylvestris). He thought that it was an element or a single substance.1 In 1756, Joseph Black, a Scottish physician, noticed this gas when heating calcium carbonate (CaCO3). He called it “fixed air”. He also identified it from exhaled breath and described it as denser than the air faintly acidic. In 1803, John Dalton (English chemist) proposed that this substance is comprised of one carbon atom and two oxygen atoms. Now, it is called “carbon dioxide”.
Carbon dioxide is a chemical compound made up of a carbon atom and two oxygen atoms. Each of the oxygen atoms is attached to the central carbon atom by a double covalent bond. The C-O bond has a length of 116.3 pm. The configuration of the compound is linear and centrosymmetric. Its chemical formula is CO2.
Carbon dioxide is a colorless, odorless, incombustible gas. Apart from gas, carbon dioxide may also occur in liquid and solid states. Dry ice is a frozen carbon dioxide. It sublimes at -78.5°C at normal atmospheric pressure.
A general depiction of an inorganic compound is one that lacks carbon atoms and archaically, not produced by a living thing (Vitalism). Nevertheless, carbon dioxide is an exception to this generalized rule. Carbon dioxide has a carbon atom and can be produced by a living thing through respiration. However, it does not have C-C and C-H covalent bonds present in organic compounds.
Due to the linearity and centrosymmetry of the carbon dioxide, this compound is nonpolar. There is no unequal sharing of valence electrons. The two oxygen atoms have equal force or electronegativity as they pull the electron density from the carbon at 180°. Thus, no net shifting of electrons occurs in any direction and as such there is no net charge build up on any of the atoms.2
Carbon dioxide is soluble in water, however, only when pressure is maintained. When it reacts to water, it forms carbonic acid, which is a weak acid. The chemical reaction is as follows: CO2 + H2O ⇌ H2CO3. Thus, carbon dioxide is present not just in the Earth’s atmosphere but also in oceans, seas, rivers, lakes, groundwater, glaciers, and ice caps. The liquid carbon dioxide returns into its gaseous state when the pressure drops, i.e. at pressures below 5.1 atm.
Carbon dioxide has a molar mass of 44.01 g·mol−1. Its melting point is -56.6 °C. At standard temperature and pressure, its density is 1.98 kg/m3. Its specific gravity is 1.53 at 21 °C. It is a nonflammable gas.
Carbon Dioxide in the Carbon cycle
Carbon is the fourth most abundant element in the Universe. The carbon cycle is a biogeochemical cycle depicting carbon exchanges on Earth. Carbon cycles through the Earth’s lithosphere, hydrosphere, and atmosphere.
In the atmosphere, carbon exists mainly in the form of carbon dioxide and methane. These two are the major factors responsible for the greenhouse effect. Although carbon dioxide is just next to methane in terms of producing a greenhouse effect per volume, it is longer-lived than methane and occurs in a much higher concentration. Thus, carbon dioxide is considered an important greenhouse gas, more than methane. The concentration of carbon dioxide in the atmosphere has risen through the years. One of the major factors that led to this rise is human activities. At the onset of the Age of Industrialization, the concentration of carbon dioxide in the atmosphere increased to about 43%. This is largely due to the combustion of fossil fuels. Also, deforestation added up to it. Trees and other photosynthetic organisms serve as the major sink of carbon dioxide. Without them, carbon dioxide would not be utilized at most and thereby accumulate in the atmosphere. Other human activities are biomass burning and cement production. Human activities are a major source of carbon dioxide on Earth. They account for about 29 billion tons of carbon dioxide per year.
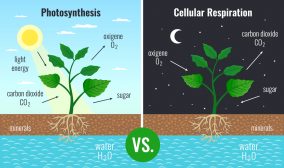
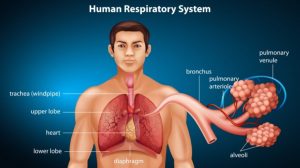
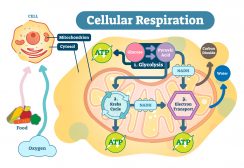
Other natural, biogenic sources of carbon dioxide are biological processes such as respiration and organic matter decomposition. In cell respiration, oxygen is taken in to be metabolized so that chemical energy (e.g. ATP) may be produced. One of the metabolic byproducts of cell respiration is carbon dioxide. In humans and other animals, carbon dioxide is collected from cells and tissues to be ultimately released outside the body by breathing it out. Carbon dioxide may also be derived from the process of decomposition or decay of organic matter.
Apart from these biogenic sources, carbon dioxide is produced from other natural sources such as volcanoes, hot springs, and geysers. Volcanoes, in particular, emit about 0.2 to 0.3 billion tons of carbon dioxide per year. Carbonate rocks dissolved in water and acids are also a source of carbon dioxide. Carbon dioxide dissolves into various bodies of water at pressures above 5.1 atm. It returns into the atmosphere as gas when pressures drop.
Biological Importance
Carbon dioxide is one of the major inorganic compounds essential to life. In animals, carbon dioxide is a chemical compound that accumulates in the tissues and removed from the body when an animal exhales. It is therefore a metabolic byproduct of carbohydrate metabolism, particularly cellular respiration wherein carbohydrate or lipid is metabolized to synthesize metabolic energy such as ATP.
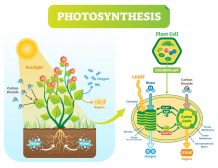
Photoautotrophic organisms such as plants and cyanobacteria use carbon dioxide as an important reactant to produce sugars by photosynthesis. The chemical reaction of photosynthesis is as follows:
n CO2 + n H2O → (CH2O)n + n O2
Photosynthesis is the primary means to remove carbon dioxide from the atmosphere.
Carbon Dioxide Uses
Carbon dioxide is used in the food industry, particularly in the manufacture of carbonated beverages. It is also used as a coolant and a refrigerant. It is also an effective fire extinguisher since it is denser than air and nonflammable.
Read:
- Carbon – an element
- Oxygen – properties and biological importance
- Photosynthesis – Photolysis and Carbon Fixation
- Respiration – Biology Online Tutorial
- Effect of CO2 on Growth & Development in Organisms
Reference
- Carbon Dioxide Encyclopedia.com. (2000, January 1). Retrieved from https://www.encyclopedia.com/science-and-technology/chemistry/compounds-and-elements/carbon-dioxide
- Ashish. (2017, October 26). Is Carbon Dioxide (CO2) Polar Or Nonpolar? Retrieved from https://www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html
© Biology Online. Content provided and moderated by Biology Online Editors