
Valine
n., plural: valines
[ˈveɪliːn]
Definition: one of the essential amino acids
Table of Contents
Amino acids are categorized into two groups based on the capability of the human body to synthesize them; essential and non-essential amino acids. Essential amino acid is the one that can’t be synthesized by the human body whereas non-essential acid can be synthesized by the human body. For detailed differences between the two types of amino acids, refer to our article on alanine. There is another way that we can categorize amino acids; branched chain and unbranched amino acids. Three amino acids belonging to the former category are valine, leucine, and isoleucine. In this article, we will focus on one of the three branched-chain amino acids, i.e., valine.
Valine Definition
Valine is an essential amino acid that is encoded by GUU, GUC, GUA, and GUG. Its solubility at 25°C is 58500 mg L-1. Its molecular weight is 117.15. Let’s discuss in detail why valine is:
- An aliphatic amino acid: Because its hydrocarbon chain is branched and without an aromatic ring structure.
- A non-polar amino acid: Because its functional group is uncharged at the physiological pH (at physiological pH, valine charge=0). Also, it doesn’t participate in any hydrogen bonding (H-bonding) at physiological pH.
- A non-aromatic amino acid: Because it comprises no aromatic ring structure. Valine doesn’t absorb UV light at 250 nm and hence doesn’t display the phenomenon of fluorescence.
- A branched-chain amino acid: Because it comprises a branch in the aliphatic side chain.
- An essential amino acid: Because it can’t be synthesized by the human body. There are 20 amino acids (organic compounds with amine, carboxyl functional groups, and side chains), out of which 9 are essential.
- An L alpha (α-) amino acid: Because the amino group is attached to the alpha-position of the carboxylic acid. In L-valine, L signifies the laevorotatory nature of valine.
- A glycogenic amino acid: Because it can be converted to glucose via the gluconeogenesis process in the liver.
- A hydrophobic amino acid: Because it has a hydrophobic side chain. This leads to a small dipole moment, thus showing repulsion to water.
- A proteinogenic amino acid: Because it plays a role in protein synthesis, thereby being highly important for muscle growth and muscle tissue recovery.
History and Etymology
Discovery: Valine (Val/V) was first isolated from casein (a milk protein) in 1901 by German chemist Hermann Emil Fischer.

Etymology: Valine derives its name from “valeric acid”. And valeric acid has been named after the plant Valeriana officinalis (common name: “valerian”) due to the presence of valeric acid in its roots.

Valine is an aliphatic, non-polar, non-aromatic, branched chain, glycogenic, essential, and alpha (α-) amino acid that is not synthesized by the human body. It is extremely hydrophobic in nature and generally found inside globular proteins. It helps in the determination of the 3-D structure of proteins. It is represented by the symbol “V” and the 3-letter code “Val”.Formula: C5H11NO2
Nomenclature
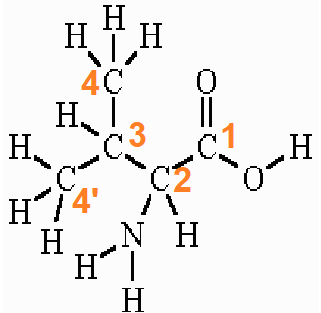
The IUPAC name of valine is valine and the other names are (2S)-2-amino-3-methyl butanoic acid or 2-Aminoisovaleric acid or valic acid. It is represented by the symbol “V” and the 3-letter code “Val”.
According to IUPAC, the carboxyl carbon is numbered 1. The α-carboxylic acid group exists in a deprotonated form (−COO–) form in normal physiological/biological conditions.
The two terminal methyl carbons (of the branch) are numbered 4 and 4′. The amino group is attached to the alpha carbon which is numbered 2. The amino group of valine exists in a protonated form (−NH3+) in normal physiological/biological conditions.
Metabolism
Valine, being one of the three branched-chain amino acids, has a different metabolism than other amino acids (essential) inside the body. The enzymes that are responsible for the initial catabolism of valine are found primarily in the extrahepatic tissues. Let’s look briefly at both the anabolism and catabolism (the 2 metabolic pathways) of valine.
-
Source and biosynthesis
Source: Plants (Reason: It can’t be synthesized in animals)
Daily requirement of dietary valine of human beings/dietary reference intakes: 24 mg/kg body weight
Biosynthesis: In both plants and bacteria
Steps involved in biosynthesis: The synthesis of valine begins from pyruvate.
- Pyruvate (pyruvic acid) is converted to acetolactate by acetolactate synthase (also called acetohydroxy acid synthase).
- Acetolactate is converted to dihydroxy isovalerate by acetohydroxy acid isomeroreductase (also called ketoacid reductoisomerase).
- Dihydroxyisovalerate is converted to 2-ketoisovalerate by dihydroxy acid dehydratase (also called dihydroxy acid dehydratase).
- 2-ketoisovalerate is converted to valine by valine aminotransferase (also simply called aminotransferase).
Within this anabolic process, there is a deviation in leucine production (another branched-chain amino acid). 2-ketoisovalerate, by the action of isopropyl malate synthase, deviates into the leucine synthesis pathway. There are 3 pyruvate family amino acids namely, valine, leucine, and alanine.

-
Degradation
The degradation of valine is similar to other branched-chain amino acids BCAAs. Their catabolism starts in the muscles. This yields NADH and FADH2. Both are utilized in the process of ATP generation.
The enzymes used in the first two steps of the catabolism of the BCAAs are the same. yielding the three different CoA derivatives. Subsequently, the metabolic pathways diverge, producing many intermediates. The principal product from valine is propionyl-CoA, the glucogenic precursor of succinyl-CoA.
- Step-1 (TRANSAMINATION): The catabolic process begins with the transamination process; removal of an amino group and formation of alpha-ketoisovalerate/alpha-ketoisovaleric acid (which is an alpha-keto acid) mediated by a BCAA aminotransferase (branched chain aminotransferase). Here, a-ketoglutarate acts as an amine acceptor.
- Step-2 (OXIDATIVE DECARBOXYLATION): Alpha-ketoisovalerate is converted to iso butyryl-CoA through oxidative decarboxylation process mediated by a branched-chain a-ketoacid only one dehydrogenase enzyme complex. There is only one dehydrogenase enzyme for all BCAAs catabolism. All three a-keto acids are acted upon by the same dehydrogenase enzyme complex. The CoA derivative formed in the catabolism of valine is therefore “isobutyryl-CoA”. The metabolic pathways diverge for BCAAs after this step.
- Step-3 OXIDATION and REARRANGEMENT: Isobutyryl-CoA is further oxidized and rearranged to succinyl-CoA. This succinyl-CoA then enters the citric acid cycle. No acetyl-CoA is formed in the catabolism of valine in contrast to the catabolism of other BCAAs (leucine and isoleucine).

Figure 5: Catabolism process of valine, leucine and isoleucine. Image Credit: Volume 1 ed. Friedman M, editor. Boca Raton, FL: CRC Press; 1989.
Synthesis
Organic synthesis of valine can be done by the “bromination process”. Isovaleric acid can be brominated as the first step. This is the followed by amination of the α-bromo derivative of isovaleric acid to yield racemic valine.
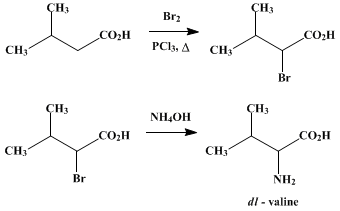
Watch this vid about valine synthesis:
Medical Significance
Valine is an essential amino acid that animals and human beings can’t produce. This necessitates the dietary intake of valine in the form of plant and animal produce. The highest amount of this essential amino acid is found in Helianthus annuus (sunflower), Cucurbita foetidissima (buffalo gourd), Moringa oleifera (moringa/drumstick), Nasturtium officinale (watercress) and Pisum sativum (pea).
Valine serves a role in normal mental health and vigor, emotional calm, optimal growth (promotes muscle growth), tissue injury repair, muscle coordination, fatigue prevention, endurance and exercise performance, increased growth hormone production, etc. This makes valine a very important constituent of the human diet.
Due to its vitality, it’s of great medical significance. Let’s discuss a few examples.
-
Brain and liver health
Valine has been used in several metabolic disorders like encephalopathy and chronic liver disease. Valine and other BCAAs help in reducing the uptake of aromatic amino acids in the brain in encephalopathy and raising the minuscule circulating levels of BCAA in patients with chronic liver disease. 3-hydroxyisobutyryl-CoA (HIB-CoA) hydrolase is one of the key enzymes in valine catabolism. It has been reported a significant decrease in HIB-CoA hydrolase activity is linked to cirrhosis.
-
Muscle Building
Valine supplements are not taken to directly build muscles in strength training but for bringing more glucose to muscle tissue. BCAA dietary supplements are quite commonly suggested in gyms and muscle-building regimes for their action in muscle protein synthesis. They have a huge market in the pharmaceutical and food industry.
-
Insulin resistance
Insulin resistance is a pathological condition. In this condition, the body cells display a failure in responding normally to insulin. The insulin hormone is responsible for the metabolism of carbohydrates, body protein, and fatty acids.
Several research studies have shown a correlation between high valine diet (or high BCAA intake) and lowered insulin resistance. (Ref-5) There is evidence that a lower level of serum BCAAs is associated with decreased insulin resistance. The study also emphasized that valine alone doesn’t worsen insulin resistance. Insulin sensitivity is probably altered by a complex mechanism involving all the BCAAs and not valine alone.
In contrast, some other studies (Ref-6) have shown a direct relation between valine to the condition of insulin resistance. There is evidence of higher levels of valine in the blood serum samples of diabetic mice, rats, and humans (elevated blood sugar levels).
-
Hematopoietic stem cells
Through the process of hematopoiesis, new blood cells are formed from hematopoietic stem cells (HSCs). The dietary source of valine plays an indispensable role in the self-renewal mechanism of these HSCs. (Ref-7) Studies have shown evidence in mouse bone marrow that any discrepancies in the valine intake can lead to the depletion of long-term repopulation of HSCs.
Case Study: “Maple Syrup Urine Disease”
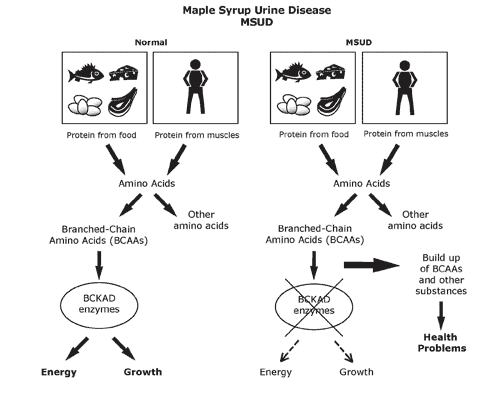
Maple syrup urine disease (MSUD) is a rare inherited genetic disorder. It leads to a serious condition where a person’s body fails to metabolize certain amino acids (BCAAs-valine, isoleucine, and leucine) to a level where it becomes harmful. This results in an escalated amount of these BCAAs in the blood and urine. The condition is named after the sweet odor resulting from the build-up of BCAAs in urine.
The faulty catabolism of the BCAAs is most commonly associated with some defect in the “branched-chain a-keto acid dehydrogenase” enzyme. Due to the integral and dedicated solo role of this enzyme in the catabolism of BCAAs, any defect in this enzyme can become lethal. MSUD is one consequential condition of such an effect. Since the enzyme is defective, all three a-keto acids accumulate (including “alpha-ketoisovaleric acid from valine catabolism”) and are excreted in the urine.
For screening for this disorder, the baby’s heels are usually pricked on the 5th day after birth to collect blood samples. MSUD condition is generally detected early on after birth and its treatment continues for life. Some of the serious symptoms of MSUD are:
- Seizures
- Normal developmental delays
- Weight loss
- Brain damage
- Poor appetite
- Recurrent episodes of metabolic crisis during lifetime (accompanied by vomits, lack of energy, irritation, and difficulty in breathing)
- Poor formation of myelin in the CNS.
Take the Valine – Biology Quiz!
References
- Torii, K. A. Z. U. O., & Iitaka, Y. (1970). The crystal structure of L-valine. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 26(9), 1317-1326.
- Hoffer, L. J., Taveroff, A. R. L. E. N. E., Robitaille, L. I. N. E., Hamadeh, M. J., & Mamer, O. A. (1997). Effects of leucine on whole-body leucine, valine, and threonine metabolism in humans. American Journal of Physiology-Endocrinology and Metabolism, 272(6), E1037-E1042.
- Letto, J., Brosnan, M. E., & Brosnan, J. T. (1986). Valine metabolism Gluconeogenesis from 3-hydroxybutyrate. Biochemical Journal, 240(3), 909-912.
- Adams, P. T., & Tolbert, B. M. (1952). A synthesis of valine. Journal of the American Chemical Society, 74(24), 6272-6273.
- Rivera, M. E., Lyon, E. S., Johnson, M. A., Sunderland, K. L., & Vaughan, R. A. (2020). Effect of valine on myotube insulin sensitivity and metabolism with and without insulin resistance. Molecular and cellular biochemistry, 468(1-2), 169–183. https://doi.org/10.1007/s11010-020-03720-y
- Lynch, C. J., & Adams, S. H. (2014). Branched-chain amino acids in metabolic signaling and insulin resistance. Nature Reviews Endocrinology, 10(12), 723-736.
- Taya, Y., Ota, Y., Wilkinson, A. C., Kanazawa, A., Watarai, H., Kasai, M., … & Yamazaki, S. (2016). Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science, 354(6316), 1152-1155.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.


