Carbon
n,, [ˈkɑːbən]
Definition: chemical element with an atomic number of 6
Table of Contents
Carbon is one of the chemical elements found in nature. A chemical element refers to the pure substance of one type of atom. At present, 94 are natural elements whereas 24 are synthetic. Carbon is one of the most common elements in living things, together with oxygen, hydrogen, and nitrogen.
Carbon Definition
Carbon (biochemistry definition): The chemical element with an atomic number of 6, and is widely distributed forming organic compounds when combined with hydrogen, oxygen, etc. Etymology: Latin carbō (“charcoal”, “coal”). Symbol: C
Properties of carbon
Carbon is the sixth element in the periodic table. Its atomic weight is 12.011. Its symbol is C. Its sublimation point 3642 °C. Carbon is a nonmetal. Its atomic number is 6. It belongs to group 14 of the periodic table. It can occur as an atom or as a pure element. Depending on the structural configuration, the allotropes of carbon will vary morphologically. For instance, graphite is opaque and black whereas diamond is transparent. Carbon is tetravalent, meaning it has a valence of four electrons. It can form so many compounds that it is referred to as the king of the elements.
Discovery of carbon
Carbon is a polyatomic nonmetal, sometimes considered a metalloid element. It was first discovered by Egyptians and Sumerians (3750 BCE)1 but it was first recognized as an element by Antoine Lavoisier (1789)2.
Carbon-containing Substances
Carbon is one of the most abundant elements on Earth. It has three naturally-occurring isotopes: Carbon-12, Carbon-13, and Carbon-14. Carbon may interact with other carbon atoms and form allotropes. It may also interact with atoms or groups of atoms of different elements to form compounds. Carbon-containing compounds may be classified as organic or inorganic.
Isotopes
Isotopes refer to any of the two or more forms of the same element. They have the same number of protons but differ in the number of neutrons in their nuclei. Therefore, they differ in atomic mass number. Nevertheless, they display almost identical chemical properties. Carbon isotopes that occur naturally include Carbon-12, Carbon-13, and Carbon-14. Carbon-12 and Carbon-13 are stable isotopes. Carbon-14 is a radioisotope, i.e. a radioactive isotope. A radioisotope decays into other elements by a process called radioactive decay. The process occurs when the unstable atomic nucleus of the radioisotope loses energy by emitting radiation. Carbon-14 is a beta emitter with a half-life of about 5,715 years. It is widely used as a tracer in studying various aspects of metabolism. This naturally-occurring isotope from cosmic ray bombardment may be used to date relics containing natural carbonaceous materials. Carbon-11 is a synthetic isotope; it is cyclotron-produced. It is a positron-emitting radioisotope of carbon with a half-life of 20.3 minutes that is used in positron-emitting tomography.
Allotrope
An allotrope of an element pertains to any of the multiple substances formed by only one type of element. The allotropes though could differ in terms of structure. For instance, coal, graphite, and diamonds are allotropes of carbon. Although they are made up of only one type of element, which is carbon, they differ in physical properties. For instance, graphite is opaque whereas diamond is transparent. Graphite is soft whereas diamond is regarded as the hardest naturally-occurring substance. Graphite is a good electrical conductor whereas diamond is not. Allotropes though are not compounds but pure elements.
Organic compounds
Organic compounds are initially defined as those substances produced only by living organisms. However, this definition was later considered as erroneous as there are substances that can be produced by both living and inanimate things. Thus, an organic compound was eventually defined as “one that contains carbon covalently bound to other atoms”, especially Carbon-Carbon (C-C) and Carbon-Hydrogen (C-H) (as in hydrocarbons). There are many ways to classify organic compounds. One of them is based on how they are synthesized. A natural organic compound (or simply natural compound) is one that is produced naturally, such as by plants or animals. A synthetic organic compound (or synthetic compound) is an organic compound that is prepared by chemical manipulations (chemical reactions). Examples of natural organic compounds are sugars, enzymes, hormones, lipids, antigens, fatty acids, neurotransmitters, nucleic acids, proteins, peptides, amino acids, vitamins, lectins, some alkaloids, and terpenoids, etc. Examples of synthetic organic compounds are synthetic polymers such as plastics and rubbers.
Inorganic compounds
A general depiction of an inorganic compound is one that lacks carbon atoms and archaically, not produced by a living thing. Later, it is defined as a compound that lacks C-C and C-H covalent bonds. Some of the carbon-containing compounds identified as inorganic are carbonates, cyanides, cyanates, carbides, thiocyanates, carbon monoxide, and carbon dioxide.
Carbon Cycle
Carbon is the fourth most abundant element in the Universe, after the elements hydrogen, helium, and oxygen. As for the Earth’s crust, carbon is the 15th most abundant element. Thus, carbon is widely circulated and converted from one form to another. Carbon cycle is one of the biogeochemical cycles that occur on Earth. Carbon cycles through lithosphere, hydrosphere, and atmosphere.
In the atmosphere, carbon exists mainly in the form of carbon dioxide and methane. These two are the major factors responsible for the greenhouse effect. Carbon dioxide is considered an important greenhouse gas, more than methane. The concentration of carbon dioxide in the atmosphere has risen through the years and one of the major factors that led to this rise is human activities, e.g. combustion of fossil fuels, concrete manufacture, deforestations, etc.
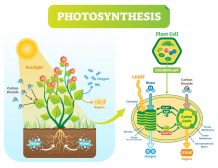
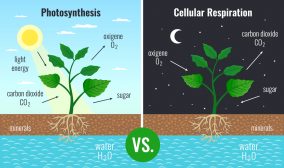
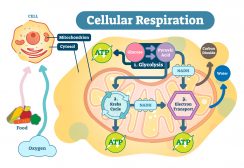
In terrestrial conditions, the amount of carbon is relatively constant. Carbon occurs and is stored in organisms (approximately 500 gigatons of carbon) and in soils (about 1500 gigatons of carbon). Organic carbon is utilized by living organisms, particularly by photoautotrophs. Photoautotrophic organisms such as plants and cyanobacteria use carbon dioxide as an important reactant to produce sugars through photosynthesis. Since living things are comprised of carbon-based compounds, they are broken down into smaller, simpler compounds through decomposition when they die. Living organisms also excrete or secrete material that is considered an organic material (matter). An organic matter pertains to any of the carbon-based compounds found in nature. This organic matter from living things becomes a part of the environment. Thus, organic matter abounds in the ecosystem, e.g. soil ecosystem. It moves into the soil or into the mainstream water where it then serves as a source of nutrition to living organisms.
Apart from these biogenic sources, carbon dioxide is produced from other natural sources such as volcanoes, hot springs, and geysers. Volcanoes, in particular, emit about 0.2 to 0.3 billion tons of carbon dioxide per year. Carbonate rocks dissolved in water and acids are also a source of carbon dioxide. Carbon dioxide dissolves into various bodies of water at pressures above 5.1 atm. It returns into the atmosphere as gas when pressures drop.
Biological Importance
Carbon is often regarded as the basis of life on earth due to its chemical properties. In the human body, it is the second most abundant element by mass, after oxygen. Carbon constitutes about 18.5% of the adult human body.
Read: Chemical Composition of the Body – Organic molecules
See Also
Reference
- “History of Carbon and Carbon Materials – Center for Applied Energy Research – University of Kentucky”. Caer.uky.edu. Retrieved from http://www.caer.uky.edu/carbon/history/carbonhistory.shtml
- Senese, Fred (2000-09-09). “Who discovered carbon?”. Frostburg State University. Retrieved from antoine.frostburg.edu/chem/senese/101/inorganic/faq/discovery-of-carbon.shtml
© Biology Online. Content provided and moderated by Biology Online Editors.